
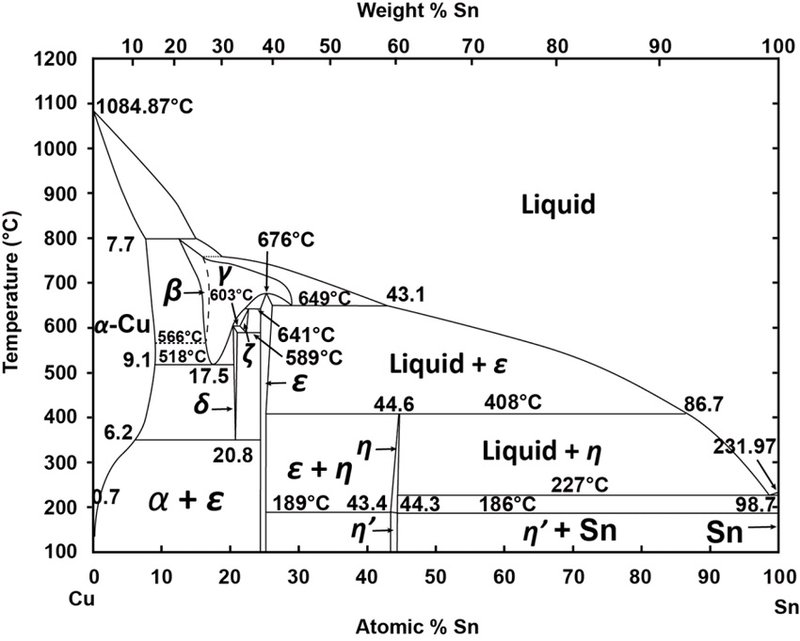
Hence, imposing tensile stress counter-balances the oxygen induced surface stress, which might have an implication on the onset of bulk copper oxidation. We also find that the thermodynamic advantage of reconstructive underpotential surface oxidation is diminished under tensile strain. By cramping into the hollow site, O ads induces compressive stress into the (100) surface, an effect that is largely absent for the adsorption at the top site. O ads at the thermodynamically favoured high-coordination hollow site (O coordinated to four Cu) is stabilised by up to 130 meV by imposing 2% tensile strain onto the surface, while the low-coordination top site (O coordinated to one Cu) shows a markedly different sensitivity. We find that different surface sites respond differently to strain. Potential-pH diagrams are also called Pourbaix diagrams after the name of their Thus, Pourbaix diagrams introduce the concept of the following three states of. Hence, additional mechanical stimuli might have a significant impact on the onset of Cu oxidation. The effects of pH on the form in which an element in a given oxidation state exists in natural waters can be summarized with predominance diagrams such as.
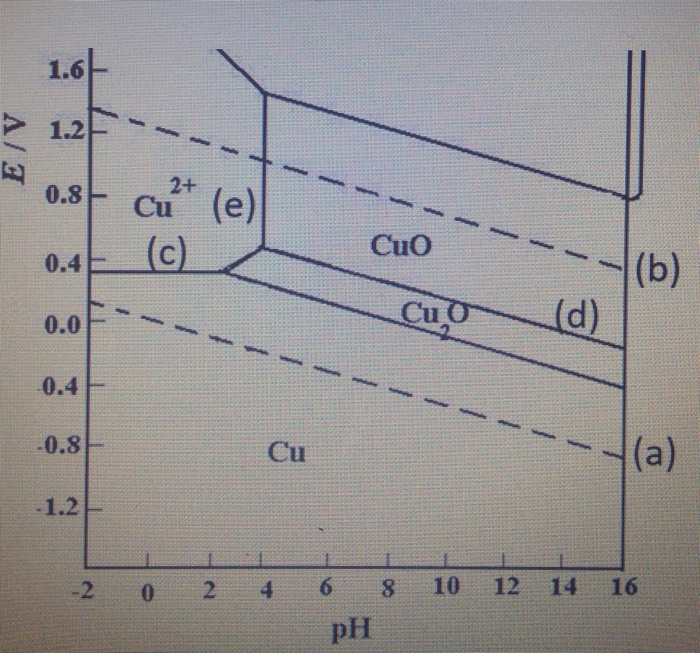

The oxidised (100) surface shows a missing-row reconstruction, which is believed to be driven by surface stress. Predominance diagrams for dissolved copper species have also been calculated. The preferred pH is between 2 and 4 (for Cu 2+ 10 -6 M), to avoid the formation of oxides. In an attempt to elucidate the relationship and underlying processes of metal oxidation under stress, we combined the electrochemical characterisation with Density-Functional-Theory (DFT) calculations to interrogate the (100) surface of copper. CHEM248: e-Lab 3 Electrodeposition & Pourbaix Diagrams Version Date: 2020.10. The Pourbaix diagram allows us to select the appropriate E and pH for copper refining, which is represented by equilibrium(1). These diagrams are often drawn assuming a dissolved copper concentration of 10 6 mol/L (mol per litre solution), which was the value selected by Pourbaix to define regions of the diagram that represented corrosion, immunity and passivation.


 0 kommentar(er)
0 kommentar(er)
